Abstract
Introduction. The histone deacetylase inhibitor (HDACi) romidepsin and the aurora A kinase inhibitor (AURKA) have both shown activity as monotherapies in patients with relapsed peripheral T-cell lymphoma (PTCL) and aggressive B-cell lymphomas (BCL), but most responses are partial and relapses often occur. In preclinical studies, AURKAs have shown to enhance lymphoma cell death to HDACis through repression of C-Myc and C-Myc-responsive micro RNAs, exhibiting highly synergistic effects in lymphoma cell lines through cytokine modulation. Therefore, we initiated a phase I trial (NCT01897012) combining alisertib with romidepsin in patients with multiple lymphoma subtypes.
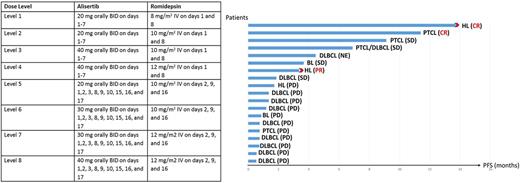
Methods. Patients with PTCL, Hodgkin lymphoma (HL) or aggressive BCL, relapsed or refractory to at least 1 line of therapy, were eligible for the study. Six dose levels were defined (Table) and a standard 3+3 design used for dose escalation; cycles were repeated every 21 days for levels 1-4, amended to every 28 days for levels 5-8, for a maximum of 8 cycles. Dose limiting toxicity (DLT) was assessed during cycle 1 and defined as grade 4 neutropenia or thrombocytopenia lasting longer than 14 days and/or uncontrollable grade 3 or 4 non-hematological toxicity.
Results. Nineteen patients have been enrolled in the study between 08/2013 and 03/2017. Median time from first diagnosis to enrollment was 11 months (range, 1-281 months); median age was 56 (range, 23-77 years) and 16 (84%) were male; 11 (58%) patients had aggressive diffuse large B-cell lymphoma (DLBCL), 3 (16%) PTCL, 3 (16%) HL and 2 (10%) Burkitt lymphoma (BL); median number or previous regimens was 3 (range, 1-8), 5 (26%) patients had received autologous stem cell transplant (SCT) and 3 (16%) allogeneic SCT; overall response rate (ORR) to latest regimen was 26% and 17 (89%) patients had relapsed within 6 months. Three patients have been enrolled at dose level 1-5, and 4 at dose level 6 (1 patient withdraw consent before completion of DLT evaluation, so cohort was expanded).
Median number of cycles delivered was 1 (range, 1-8) and 18 (95%) patients have discontinued treatment to date; reasons for study discontinuation were: progression in 14 (78%) patients, completion of treatment in 2 (12%), indication for SCT in 1 (5%), and patient's choice in 1 (5%). No DLTs have been observed to date. The most common (> 10%) grade 3-4 toxicities were thrombocytopenia (47%), anemia (32%), neutropenia (32%), infections (26%) and fatigue (16%). Fifteen patients were evaluable for response by PET/CT, and 4 patients stopped treatment after 1 cycle, before restaging: 3 because of clinical progression and 1 because of patient's choice; among 7 patients who had a decrease in tumor burden, median reduction was 44% (range, 11-89%), and among 8 patients who had an increase in tumor burden, median increase was 51% (range, 4-300%). Two patients (10%) achieved complete remission (1 patient with HL, 1 with PTCL), 1 (5%) partial remission (1 patient with HL), and 4 (21%) stable disease, and ORR rate was 15%. Only 1 (5%) patient proceeded to SCT (allogeneic) after 3 months of treatment. After a median follow-up of 4 months (range, 1-46 months), median progression-free survival (PFS) was 1 month (range, 1-14 months), and the longest PFS (> 6 months) was observed in 1 patient with heavily pretreated HL (lymphocyte depleted) and 3 patients with PTCL (Figure). At most recent follow-up, 17 patients have progressed, 1 after SCT.
After a median follow-up of 4 months (range, 1-46 months), median OS was 7 months (range, 1-46 months). At most recent follow-up, 11 (58%) patients have died, all of disease progression.
Discussion. The combination of romidepsin and alisertib can be safely used as salvage therapy in patients with relapsed or refractory aggressive B-cell lymphomas and PTCL, however the majority of patients have developed disease progression during follow-up suggesting rationale for potential maintenance therapy, improved methods to select patients who would have the highest likelihood of benefit, and evaluation of other targeted therapy doublets or triplets for these diagnoses. Initial gene expression profiling (GEP) results have shown that upregulation of genes associated with anti-tumor immune response correlates with response. Enrollment to the higher dose level (7 and 8) is ongoing.
Nastoupil: Janssen: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; TG Therapeutics: Honoraria, Research Funding; Gilead: Honoraria; Karus Therapeutics: Research Funding; Abbvie: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Kwak: InnoLifes: Consultancy, Equity Ownership; Celltrion, Inc: Consultancy; Pepromene Bio: Consultancy, Equity Ownership. Lee: KITE PHARMA: Consultancy. Wang: BeiGene: Research Funding; Celgene: Honoraria, Research Funding; Dava Oncology: Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; June Therapeutics: Research Funding; Kite Pharma: Research Funding; Onyx: Research Funding; Pharmacyclics: Research Funding; Proteolix: Honoraria, Research Funding; Acerta Pharma: Consultancy, Research Funding; Asana Biosciences: Research Funding. Westin: Apotex: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees. Wesson: seattle genetics: Honoraria. Fanale: BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CELGENE: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ONYX: Research Funding; MERCK: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC THERAPEUTICS: Research Funding; AMGEN: Membership on an entity's Board of Directors or advisory committees; MOLECULAR TEMPLATES: Research Funding; SEATTLE GENETICS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; TAKEDA: Honoraria, Research Funding; GENENTECH: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal